Air-Water Gas Transfer Studies at the Wind-Wave-Tank
(Ph.D. thesis of W. Siems; text by P.A. Lange)
This dissertation describes a study motivated by a desire to better understand the important role the ocean plays in the biogeochemical cycles of climate modifying gases such as carbon dioxide, CO2. The gas transfer velocities for CO2 and O2 were determined as a function of the windspeed and fetch by measuring their desorbtion from the gas enriched water in the wind-wave-tank.
The influence of monomolecular films on the gas exchange process was also investigated. Synthetic organic films were used as proxies for biogenic slicks which are especially prevalent coastal waters.
Breaking waves are produced in the wave-tank at windspeeds exceeding 10 m/s with the concurrent appearance of bubbles. Their size distribution was determined and their influence on the the gas exchange rate was measured.
References:
- Siems, W., 1980: Modelluntersuchungen zur Verdunstung und zum Gasaustauch zwischen Wasser und Luft. Der Einfluss von Wellen und Oberflächenverunreinigungen, Dissertation, Universität Hamburg, Fachbereich Chemie, 241pp.
Broecker, H. Ch., J. Petermann, and W. Siems, 1978: The influence of wind on CO2-exchange in a wind tunnel, including the effects of monolayers, J. Mar. Res., 36, 595-610.
Broecker, H. Ch., and W. Siems, 1984: The role of bubbles for gas transfer from water to air at higher windspeeds, in Gas transfer at Water Surfaces, Brutsaert and Jirka (eds.), D. Reidel Publishing, Dordrecht, 229-236.
Experimental Setup
The gas enrichment apparatus consisted of a network of perforated polyethelene tubing placed at the bottom of the tank and connected to the gas cylinders. Wind velocities and water wave amplitudes were measured with the Prandtl-Pitot tube and a resistance wire gauge, respectively. CO2 concentrations in the air were recorded with an infrared gas analyser; in the water using the desorbtion and pH methods.
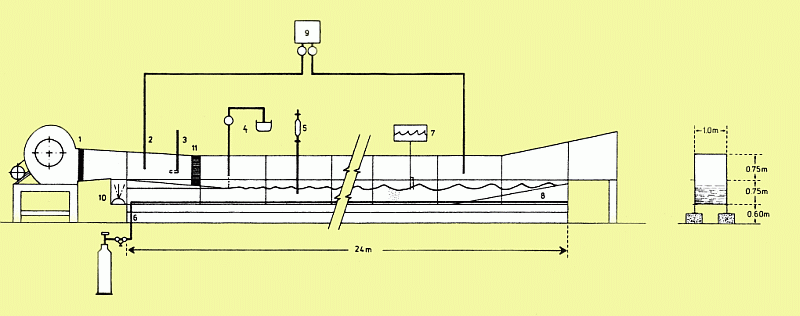
Schematical view of the experimental setup. 1: blower, 2: CO2 profile measurement, 3: Prandtl-Pitot tube, 4: monolayer deployment, 5: water sample extraction, 6: gas enrichment apparatus, 7: wave gauge, 8: beach, 9: infrared gas analyser, 10: wave flap, 11: honeycomb
Wind-Speed Dependency
The gas exchange rate was calculated from the rate of decrease and increase of CO2 and O2 concentration in the water and the air, respectively, measured at fixed locations. Note the break in the curves at 10-14 m/s wind which is concurrent with the appearance of bubbles.
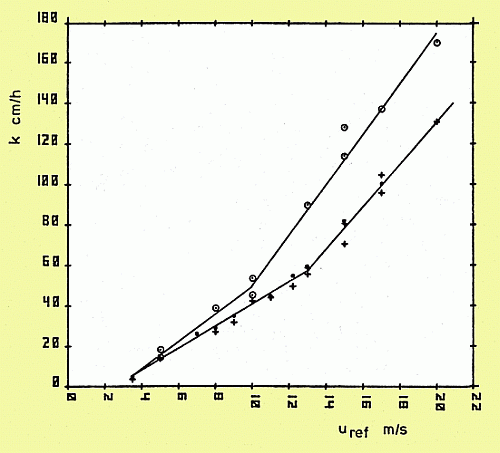
Gas Transfer Velocity vs. Windspeed: O2 (o) and CO2 (+ = pH method [water], . = partial pressure method [air])
Dependence on Slick Coverage
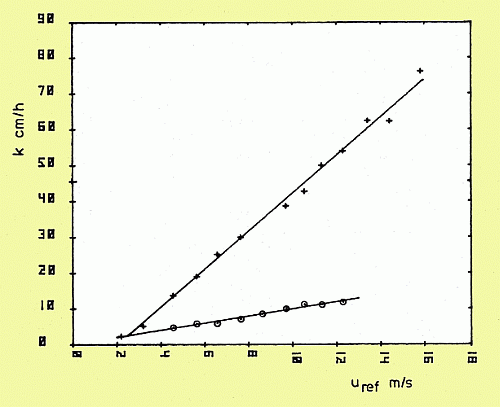
Gas exchange rate as a function of windspeed with (o) and without (+) a monomolecular oleyl alcohol film covering the water surface.
Bubble Measurements
A photographic method was chosen for determining the size distribution of entrained air bubbles.

Photograph of a train of wave-generated bubbles.
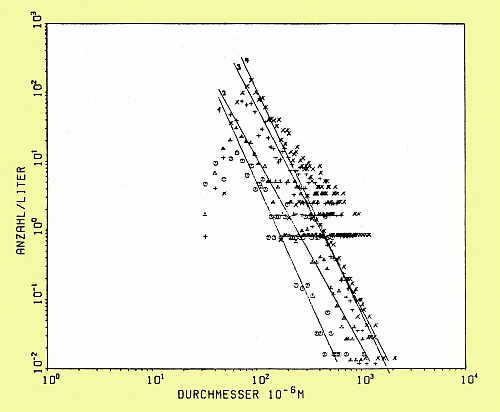
Bubble size (diameter) distribution measured at 11.5 m fetch and at 20 cm depth. Reference wind speed, U_ref = 13 m/s (o), U_ref = 15 m/s, U_ref = 17 m/s (+), U_ref = 19 m/s (x)
Wind-Wave Spectra at Various Wind Speeds
The dominant factor influencing the CO2 and O2 gas exchange appears to be the wind generated wave field. The momentum transfer from the wind to the water surface changes the dynamic characteristics and the boundary layer of the water. An increased turbulence in the water leads to an eroding of the laminar boundary layer in the water, so that diffusion processes play a reduced role in the gas transfer (today, it is known that the orbital motion of the longer waves also play an important role; "surface renewal").
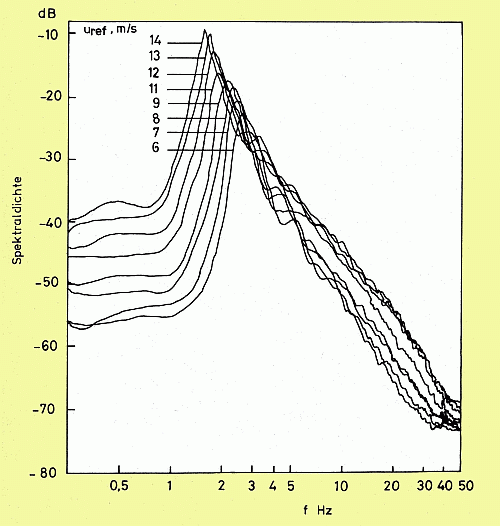
Wind-wave spectra (measured at 11.5 m fetch) at various wind speeds.
Wave Damping by Slicks
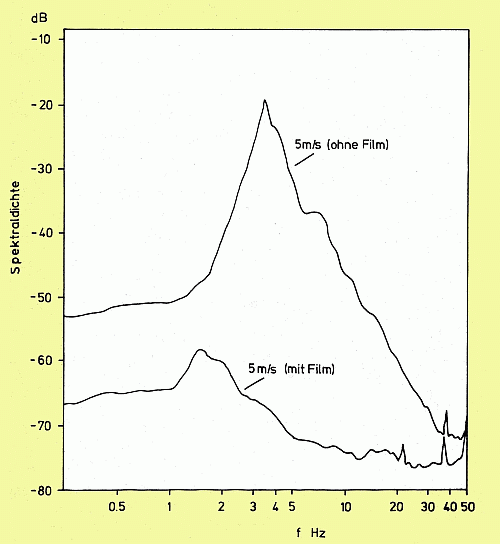
Damping of wind generated waves by an oleyl alcohol monomolecular film. Spectra were measured at 11.5 m fetch, film was deployed at 0 m fetch. Upper curve: slick-free water surface, lower curve: slick-covered water surface.
Conclusions and Recent Work
The gas exchange increases linearly with increasing windspeed (or friction velocity) as soon as initial capillary waves appear on the water surface. The onset of breaking waves beyond 10 m/s windspeeds further enhances the gas exchange through the entrainment of bubbles. Monomolecular films substantially decrease the gas exchange in that they present a chemical barrier for the gas transfer, and substantially dampen the all important wind generated capillary waves which play an essential role in the the gas exchange process.
More recent studies by many research groups have confirmed the results of this thesis [1,2]. In fact, a number of field studies have shown that the results of this and many other laboratory studies present a basically correct picture of the gas exchange dependencies. However, today, more emphasis is placed on so-called "surface renewal" concepts, where it has been shown that the mechanisms that continually transport "fresh" water from deeper layers to the air-water boundary, such as turbulence and the orbital movements induced by the long and short water waves, substantially contribute to gas transfer at the water-air interface.
Recent research was aiming to establish a link between globally distributed gas exchange rates and parameters that can be measured via satellite imaging (e.g., surface roughness due to wind-waves).
Together with the team of Bernd Jähne at the University of Heidelberg we investigated the influence of rain-induced turbulence at the water surface on the gas exchange processes (funded by the German Research Foundation).
References:
- 1. Third International Symposium on Air-Water Gas Transfer, July 24-27, Heidelberg,1995
- 2. Gas Transfer at Water Surfaces. Geophys. Monograph, American Geophysical Union, 2002
 .. back to the Wind-Wave Tank page...
.. back to the Wind-Wave Tank page... 
 .. or to the KFEW3O page ...
.. or to the KFEW3O page ...
