The Wind-Wave-Tank Chemistry Story:
(Text: H. Hühnerfuss)
Tracing back the different wave damping and remote sensing characteristics of sea slicks to their chemical properties
Man-made and natural surface films ("slicks") are widely prevalent on the world's oceans, particularly in coastal waters. For example, biogenic slicks are being secreted by plankton or by coral reefs thus causing quieter water conditions by damping dangerous water turbulence that might endanger delicate coral forms or crash plankton within the upper marine water surface. The most notable geophysical characteristic of these slicks is their ability to dampen both capillary water waves and short gravity water waves. As a consequence, modification of gas exchange as well as of the signals of various remote sensors are being induced. Therefore, the main goals of the present work are:
- the elucidation of the water wave damping mechanism caused by monomolecular sea slicks
- the correlation of the wave damping effects with the chemical structure of the film-forming substances
- tracing back the wave damping effects to specific chemical parameters of the respective film-forming substances
- the correlation of the modification of further air-sea interaction processes, such as gas exchange, and of remote sensing signals with the chemical properties of the film-forming substance
Model Substances
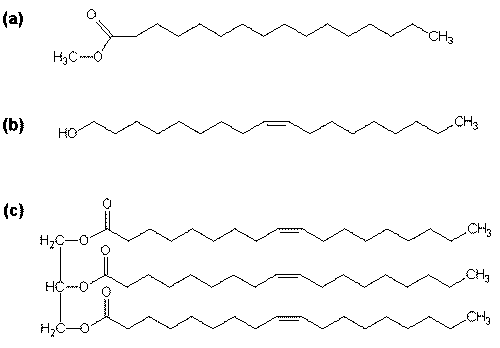
The chemical analysis of biogenic and man-made sea slicks suggested that typical characteristics are prevalent including the presence of a long alkyl chain (hydrophobic tail) and a polar head group (hydrophilic part). These elements are exemplarily represented by constitutional formulae of a) hexadecanoic acid methyl ester (Palmitic acid methyl Ester = PME), b) Z-9-octadecen-1-ol (oleyl alcohol = OLA), and c) triolein (TOLG).
Correlation of water wave damping with the chemical structure
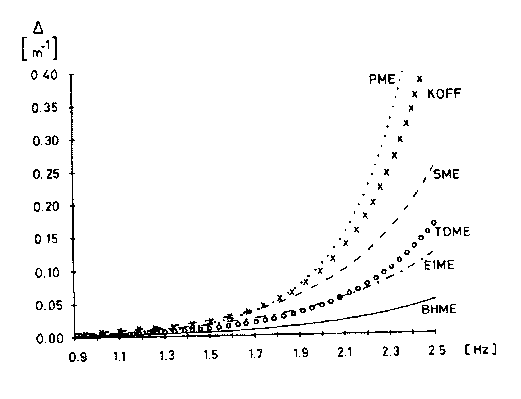
A representative result of our systematic wind-wave-tank investigations aiming at the correlation of water wave damping with the chemical structure of the film-forming substance is given for carboxylic acid esters (PME-type see above). Prolongation of the alkyl chain length between C14 (TDME) and C22 (BHME) revealed that maximum wave damping is achieved in the presence of a PME film (C16-chain). Less wave damping was observed for C18 (SME) and C14 (TDME) slicks. Basically, an increase in alkyl chain length leads to a continuous increase in the van der Waals interactions between the long chain which until a chain length of C16 is favourable for an increased wave damping effect. However, concurrently a lost of elasticity is observed as well which be- yond a chain length of C16 starts to overcompensate the favourable van der Waals gain. In detail, water wave damping is determined by Marangoni damping which in turn depends on a complex dilational modulus that comprises a real elasticity and an imaginary viscosity term. The wind wave tank experiments allowed a determination of the exact dilational moduli and thus the wave damping coefficients for nearly 100 organic surface-active compounds representing all important structures prevalent in biogenic and anthropogenic sea slicks.
Implications for the marine environment
Furthermore, it is worth noting that biogenic slicks secreted by plankton often contain large quantities of carboxylic esters consisting of about 70 to 75 % of C16 and C18 alkyl chains. In summary, "nature" is using preferentially those chain lengths for a "self-protection" of plankton that exhibit the maximum wave damping potential (see the KOFF-curve in the above Figure).
Morphology of Sea Slicks
Morphology effects reflect the fact that the same film-forming compound may be arranged and distributed at the air/water interface in the following different manners:
- The molecules may be spread homogeneously.
- The molecules may form 'islands', so-called domains, of different sizes between microns and several hundred microns in diameter.
Method
The water surface is being surveyed by 'Brewster Angle Microscopy' [BAM]. A schematic sketch of the BAM set-up is shown in the lower left-hand Figure, while the experimental approach is depicted in the lower right-hand Figure.
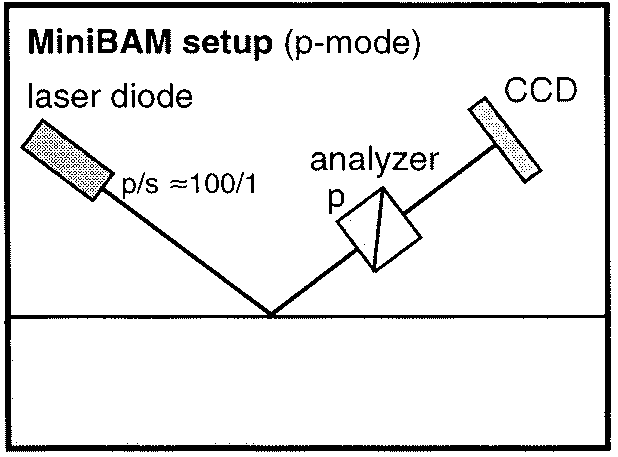
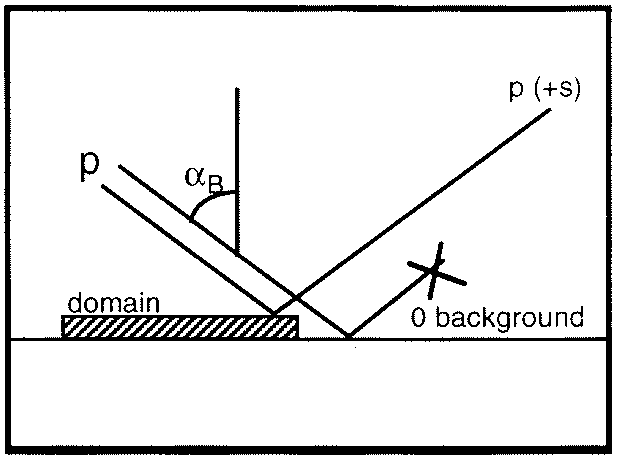
If the incidence angle of p-polarised radiation is equal to the Brewster angle, αB, the reflectivity from the pure (i.e., slick-free) water surface is close to zero. As a consequence, the viewed water surface appears to be dark. In the presence of a film-forming substance, however, the slick patches represent a different optical medium that gives rise to a measurable reflectivity, which in turn makes the slick domains visible by their lighter appearance. This effect can be recorded by a Charge-Coupled-Device [CCD]-camera.
Morphology Results
As an illustration of the versatility of BAM, examples of the investigation of two problems will be given:
- the morphology changes resulting from different compression of a slick on an undulating water surface.
- the different morphologies attained by different spreading procedures of slick-forming material.
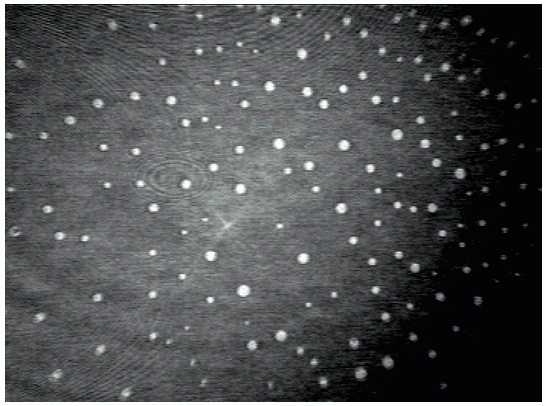
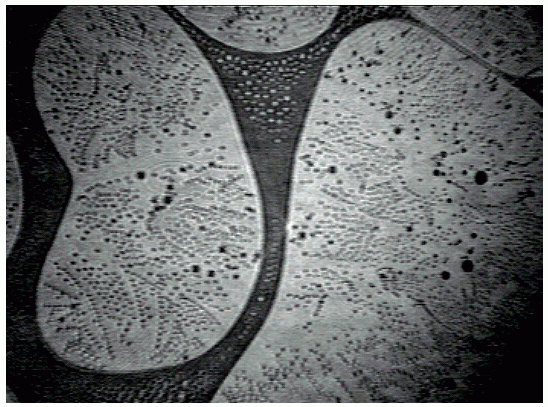
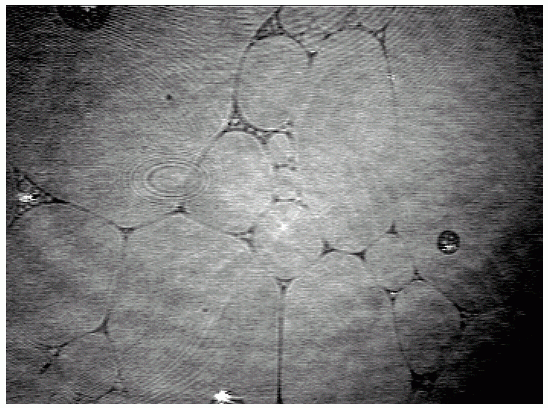
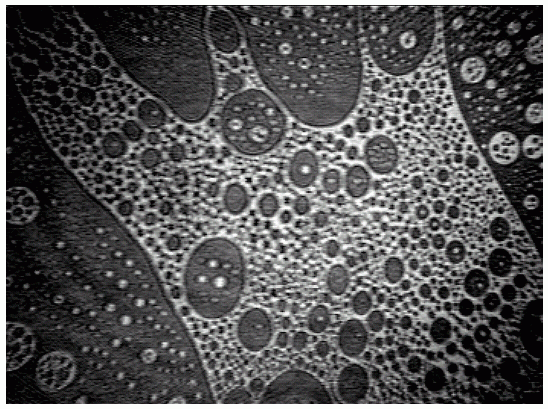
A sequence of BAM images obtained in the course of compression of a slick consisting of hexadecanoic acid methyl ester (Palmitic acid methyl ester = PME) is shown in Figs. 3-5. At large area per molecule, relatively small domains prevail (a), which, under compression, combine so that kidney-like shapes are being formed ((b) and (c)). A completely different situation is encountered when the same slick material is being spread from ethanol (d). Already at large areas per molecule a foam-like structure is being formed, i.e., a two-dimensional network which is expected to exhibit quite different viscoelastic characteristics compared to those of the morphological structures of the PME film displayed in Figs. (a)-(c).
|
|
|
|
Geophysical implications of our Morphology Results (Example PME)
The different morphology of palmitic acid methyl ester (PME) slicks spread from n-hexane or ethanol showed that in the latter case already at large areas per molecule a foam-like structure is being formed on the water surface, i.e., a two-dimensional network which appears to be comparable with the morphological structure of biogenic sea slicks. In line with this assumption, both the magnitudes of the radar backscatter damping ratios and the characteristics of the damping ratio/wavenumber curves were comparable for the PME slick spread from ethanol and for the biogenic slicks, while in the presence of the PME slick spread from n-hexane lower damping ratios were determined. In the first instance, we were able to simulate the water wave damping characteristics of the biogenic sea slicks very well.
IRRAS Relaxation Results
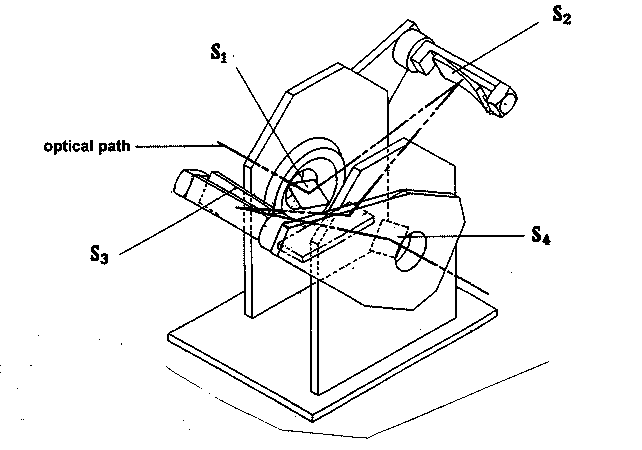
The molecular structure of the slick/adjacent water layer system is being investigated by 'Infrared Reflection-Absorption Spectroscopy' [IRRAS], a method that allows in-situ investigations of the monolayer at the air/water interface without disturbing the structure and morphology of the film.
The IRRAS study was performed on a Bruker IFS 66 spectrometer (Karlsruhe, Germany) equipped with an MCT detector and a modified external reflection attachment P/N 19650 of SPECAC (Orpington, UK). This included a miniaturised Langmuir-trough, permitting thermostatic measurements. The IRRAS set-up as well as the experimental approach can be inferred from the schematic sketch shown below.
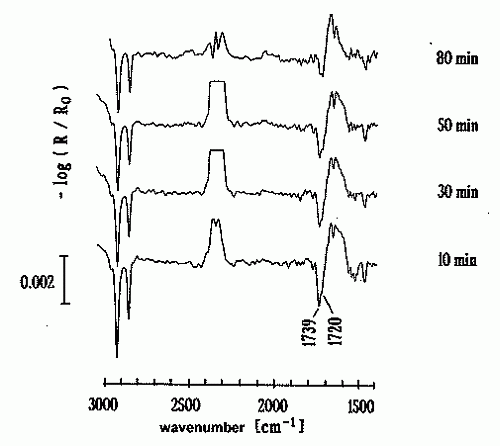
The versatility of IRRAS for the in-situ investigation of slick structures on the air/water interface is demonstrated in the figure below, which summarises the results of a relaxation experiment: a PME monolayer was generated at a water surface and then compressed to 0.272 nm2/molecule. In the course of the compression procedure, some disorder is induced within the monolayer. After stopping the compression, the molecules tend to arrange themselves such that an optimum order of the alkyl chains and of the head groups is attained. This is largely the relaxation process that is also encountered on a slick-covered undulating water surface. In detail, the IRRAS results show that alkanoic acid esters are continuously hydrated and dehydrated during compression and dilation on an undulating water wave field. It can be safely assumed that the strong water wave damping effect of these compounds is centrally related to this phenomenon.
Geophysical implications of our Morphology Results (Example PME)
The relaxation of alkanoic acid esters, which are often being found in biogenic sea slicks, was investigated by 'Infrared Reflection-Absorption Spectroscopy' [IRRAS]. It turned out that the ester group is continuously hydrated and dehydrated during compression and dilation on an undulating water wave field. It can be safely assumed that the strong water wave damping induced by these chemical compounds is centrally related to this phenomenon.
 .. back to the Wind-Wave Tank page...
.. back to the Wind-Wave Tank page... 
 .. or to the KFEW3O page ...
.. or to the KFEW3O page ...